Xupei Pu, Yongqiang Sun, Jingjie Zhou, Yuqi Liu, Jinyuan Sun, Huibin Liang, Guanjie Liu, Chunyu Wang, Ke Zhang, Martino Di Serio, Rosa Vitiello
Journal of Dispersion Science and Technology (2024)
https://doi.org/10.1080/01932691.2023.2301488
Abstract
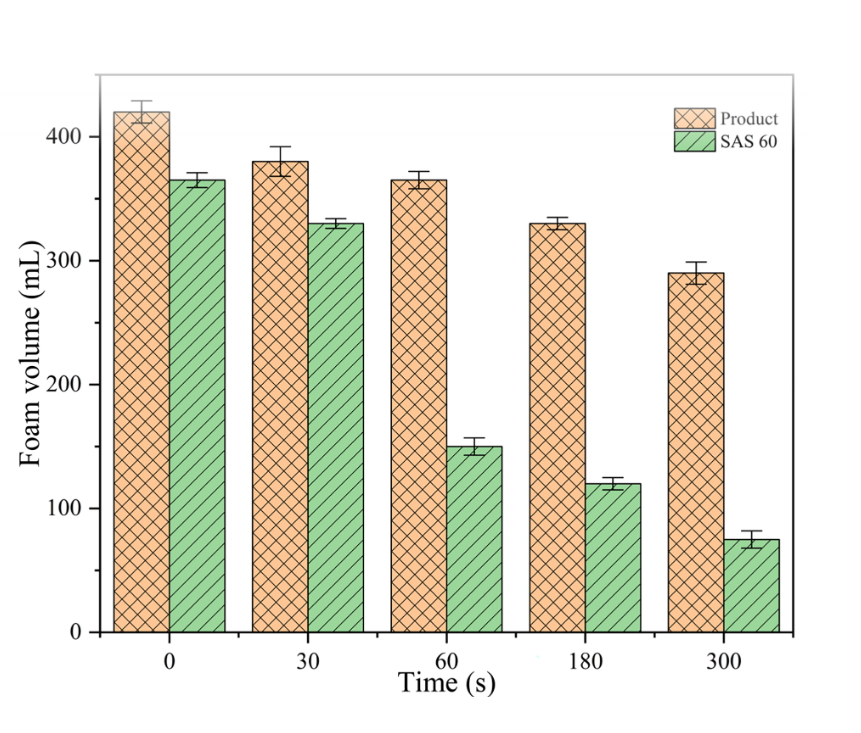
Currently, there are totaling two production methods of sodium secondary alkyl sulfonate SAS 60, that are, sulfochlorination and sulfoxidation. These two methods involving SO2 (the first one is also Cl2) may be harmful to the enviroment. In this paper, sodium secondary alkyl sulfonate was synthesized by NaHSO3 addition reaction of dodecane’s double bond with azodiisobutyronitrile as catalyst, and the yield was over 95% within 2 h. The reaction avoids SO2 and Cl2, with the preparation process being simple and safe. The target product β-sodium dodecyl sulfonate was characterized by FT-IR, mass spectrometry,1H NMR and 13C NMR characterization. The physical and chemical properties such as surface tension, contact angle, wettability, emulsification, foaming and decontamination were tested. The results demonstrate that the Krafft point for the determination of sodium secondary alkyl sulfonate is 26 °C, with its CMC of 2.93 × 10−3 mol/L and γcmc of 27.06 mN/m according to γ-lgc curve. The results of thermodynamic parameters depict that micellization of the surfactants is a spontaneous process. Compared with conventional SAS 60, sodium secondary alkyl sulfonate is an anionic surfactant with excellent emulsifying ability, favorable foam performance and corking foam stability