Turco, R.; Di Serio, M.
Sustainable Synthesis of Epoxidized Cynara C. Seed Oil.
Catalysts 2020, 10, 721
Abstract
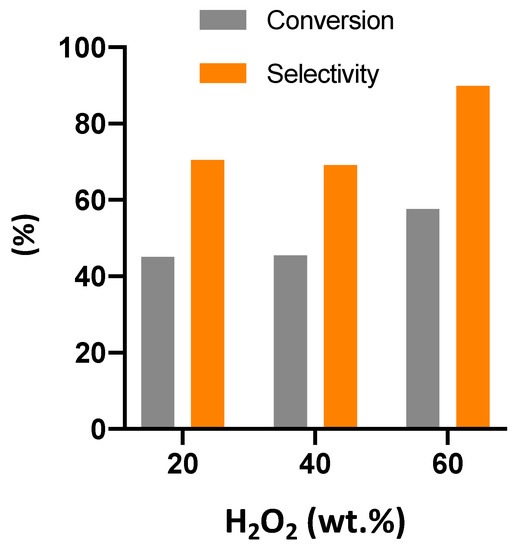
The use of non-edible vegetable oils to produce oleochemicals has been attracting more attention in recent years. Cardoon seed oil, derived from the Cynara C. plant, growing in marginal and contaminated lands, represents a non-edible alternative to soybean oil to obtain plasticizers through epoxidation reaction. The use of hydrogen peroxide as oxidant and in the presence of a heterogeneous catalyst allows overcoming the limits of epoxidation with peracids. γ-alumina has been shown to have an active catalyst epoxidation reaction with hydrogen peroxide, mainly using acetonitrile as solvent. However, the use of acetonitrile as solvent is widely debated due to its hazardous character and health issues. For these reasons, the influence of solvent on the reaction was studied in this work to find a more environmentally friendly and stable solvent. The study showed that the epoxidation reaction takes place also in the absence of solvent although with lower selectivity. The type of solvent influences both the epoxidation and decomposition reactions of hydrogen peroxide. γ-valerolactone was found to be the most promising solvent for cardoon oil epoxidation reaction. This finding represents a noteworthy novelty in the field of epoxidation of vegetable oils with hydrogen peroxide, opening the way to greener and cleaner process. Finally, an optimization study showed that the most effective molar ratio between hydrogen peroxide and double bonds for better selectivity was 4.5 and the need to use the highest possible initial concentration of hydrogen peroxide (approximately 60 wt. %).