Luca Riccio, Martino di Serio, Vincenzo Russo, Tapio Salmi
Chemical Engineering Research and Design Volume 218, June 2025, Pages 133-146
https://doi.org/10.1016/j.cherd.2025.04.038
Abstract
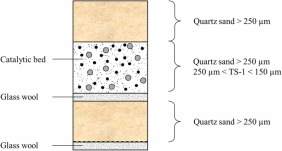
Liquid-phase epoxidation of 2-butene and equimolar mixtures of 1-butene and isobutene was studied in a laboratory-scale trickle bed reactor. Hydrogen peroxide was used as the environmentally friendly epoxidation agent and commercial titanium silicate (TS-1) as the catalyst. Traditionally, alkene epoxidation experiments have been carried out at steady state or in batch mode, but in this work, transient response experiments were conducted under isothermal conditions to retrieve very precise kinetic data. The reaction temperature and pressure were varied within the intervals 15–40 °C and 1 bar, respectively. The highest butene conversions were around 85 % and the epoxide selectivity was 95 %. The experimental results were interpreted with a dynamic trickle bed reactor (TBR) model, which consisted of coupled parabolic partial differential equations for the gas and liquid phases and ordinary differential equations for the solid catalyst phase. The model equations were solved numerically with the software gPROMS, to estimate the kinetic and adsorption parameters and to predict both the reactant and product concentrations. Nonlinear regression analysis was applied in the parameter estimation. A good description of the experimental data was obtained.