Modeling of microreactors for ethylene epoxidation and total oxidation.
Russo, V., Kilpiö, T., Hernandez Carucci, J., Di Serio, M. & Salmi, T. O.
Chem. Eng. Sci. 134, 563–571 (2015).
Abstract
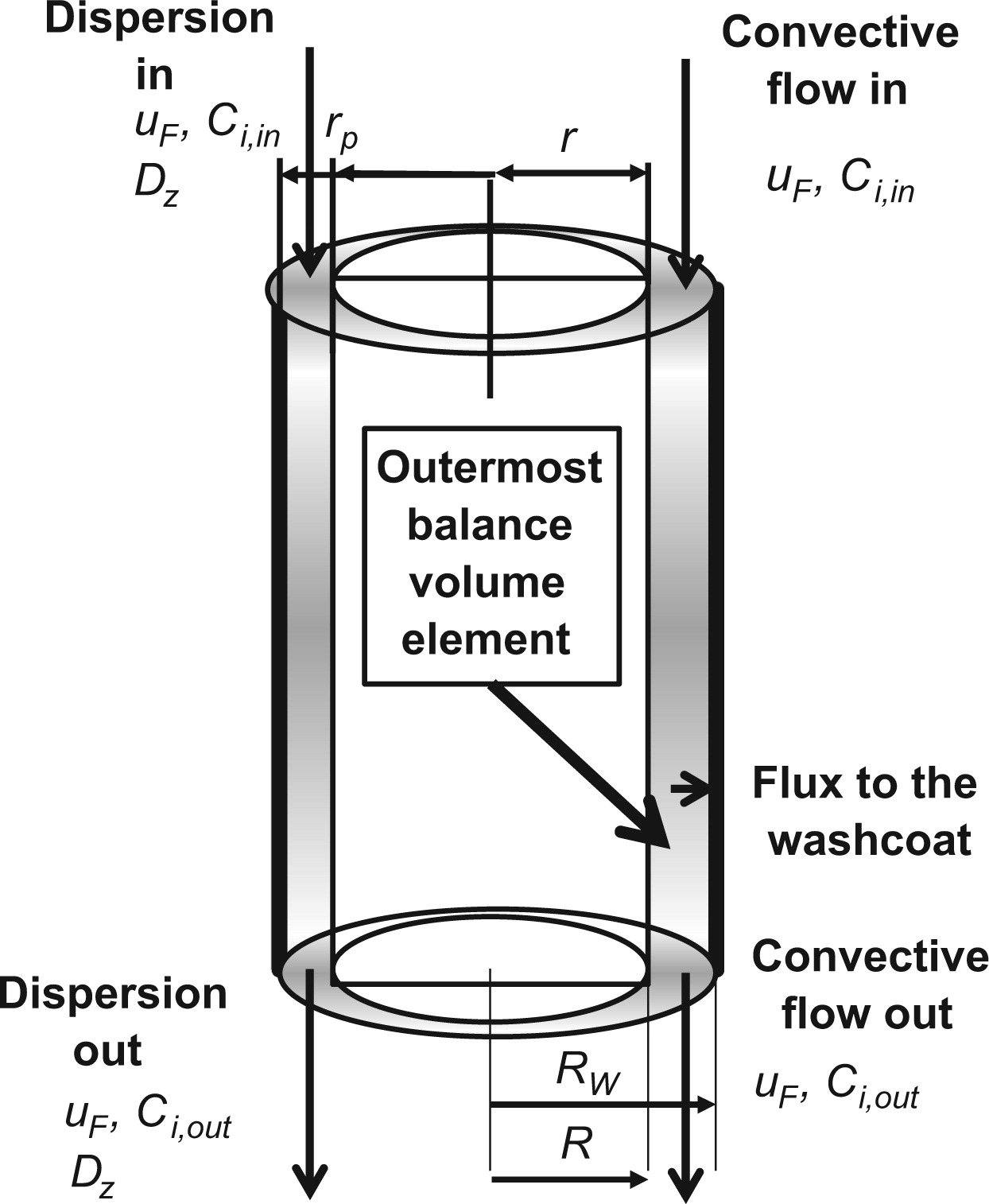
Microreactors are especially well-suited for laboratory-scale studies of rapid exothermic reactions, because they have excellent mass and heat transfer characteristics. Two novel reactor models were used for plate microreactors, one for a washcoated reactor and the other one for a silver plate microreactor. The aim of this modeling study was to precisely explain both the concentration and the temperature dependencies of the reaction rates of ethylene oxide (EO) synthesis over a wide range of operating conditions (p, T, flows) by using all the extensive data generated with our microreactors. Microreactors are especially well suited for producing accurate kinetic data because of the uniformity of reaction conditions in the reactor system.
Modeling took into account the reaction and mass transfer effects. The axial and radial concentration profiles and for the washcoated case also the concentration profiles along the coating direction were solved numerically. The models were based on dynamic mass balances for the gas phase, with convection, axial and radial dispersion terms included. From the modeling viewpoint, an interesting phenomenon, the interaction between intrinsic kinetics and diffusion in the porous catalyst layer, was tackled with the aid of mathematical modeling for the washcoated reactor case. The reactor models were solved numerically by using gPROMS software. Ethylene oxidation on silver catalyst was the selected example reaction system, because the main product, ethylene oxide, is a key compound and an important intermediate for chemical industry. The reactor and kinetic models were able to describe all the experimental data with a very satisfactory agreement. The microreactor models developed are generic and applicable to various kinds of heterogeneously catalyzed gas-phase reaction systems.
The industrial breakthrough of flow chemistry and catalyzed single phase microreactor technology is expected to take place first in the production of fine and specialty chemicals. For some bulk products (e.g EO), flow chemistry has already arisen interest. The benefits that microreactors can offer are the exact control of process conditions, the compact reactor size and the ease of scalability by simply “numbering up”. For a new product, ease of scalability shortens the time to market, whereas for a bulk product like EO, millireactors (structurally similar to microreactors, just having larger flow channels) may be more potential candidates for on-site production applications.