Liquid–Liquid–Solid Model for the Epoxidation of Soybean Oil Catalyzed by Amberlyst-16
Martino Di Serio, Vincenzo Russo, Elio Santacesaria, Riccardo Tesser, Rosa Turco, and Rosa Vitiello
Ind. Eng. Chem. Res. 2017, 56, 45, 12963–12971
Abstract
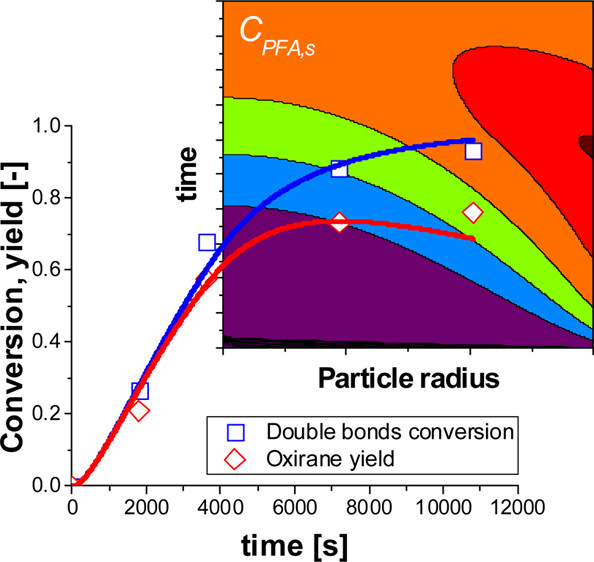
The epoxidation of soybean oil is a hot topic for biorefinery. The process is nowadays performed in the presence of a strong mineral acid (H2SO4, H3PO4) that catalyzes both the epoxidation and the oxirane ring opening reactions, leading to high conversions and low selectivity. Moreover, the separation of such a catalyst needs neutralization steps, thus operation units. Previous studies revealed that Amberlyst-16, an acid exchange resin, is a stable and active catalyst in the epoxidation of soybean oil, showing high selectivity. In the present paper, a rigorous liquid–liquid–solid kinetic model has been proposed to interpret the kinetics of the soybean oil epoxidation reaction catalyzed by Amberlyst-16. The intrinsic kinetics of the overall reaction network has been investigated by taking into account all the occurring chemical and physical phenomena, overall dealing with the intraparticle diffusion limitations. From the data elaboration, it has been demonstrated that the unique role of the resin is to catalyze the formic to performic acid step. Formic acid showed a higher rate in decomposing the oxirane ring with respect to performic; this was justified by its higher acidity. The high selectivity is due to the fact that the resin has no action in catalyzing the ring opening reactions because of its high hydrophilicity. The obtained results are a good starting point for designing a continuous reactor.