Lactic Acid-Based Solvents for Sustainable EDLC Electrolytes
Massimo Melchiorre, Roberto Esposito, Martino Di Serio, Giancarlo Abbate, Alessandro Lampasi,
Andrea Balducci and Francesco Ruffo *
https://www.mdpi.com/1996-1073/14/14/4250/htm
Abstract
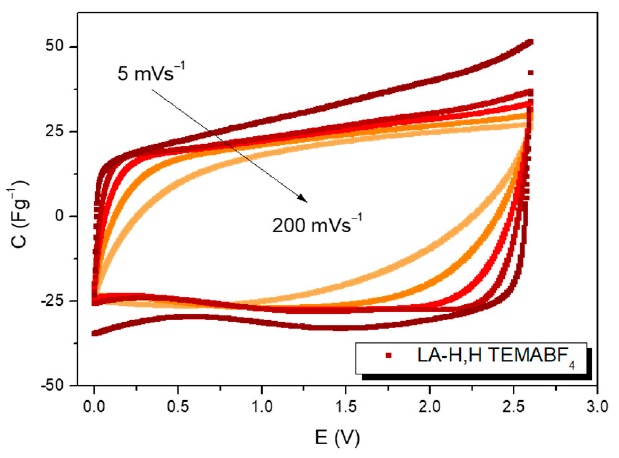
The most relevant electrolytes used in commercial electrical double layer capacitors (EDLCs) are based on non-aqueous solvents as acetonitrile (ACN) and propylene carbonate (PC). However, these solvents are synthesized from non-renewable fossil feedstocks, making it desirable to develop more sustainable alternatives. To address this issue, in this work lactic acid was used to synthesize a panel of substances with small structural variation. The investigated products belong to the chemical family of ketals, and among them the 5-methyl-1,3-dioxolan-4-one (LA-H,H) was found to be the most suitable to prepare electrolytic solutions. Therefore, LA-H,H was combined with triethylmethylammonium tetrafluoroborate (TEMABF4), and analyzed in symmetrical EDLC. This electrolyte was thoroughly characterized by cyclic voltammetry, galvanostatic cycles and electrochemical impedance spectroscopy (EIS), disclosing competitive performances compared to PC-based electrolyte. The EDLC with LA-H,H/TEMABF4 displayed a specific energy and powerof 13.4 Wh/kg and 22.5 kW/kg respectively, with an optimal cycling stability over 5000 cycles at different current densities.