T Salmi, WY Perez-Sena, F Ciccarelli, K Eränen, A Medina, T Cogliano, , Martino Di Serio, Johan Wärnå, Sébastien Leveneur, Vincenzo Russo
Chemical Engineering Science 285 (2024) 119578 https://doi.org/10.1016/j.ces.2023.119578
Abstract
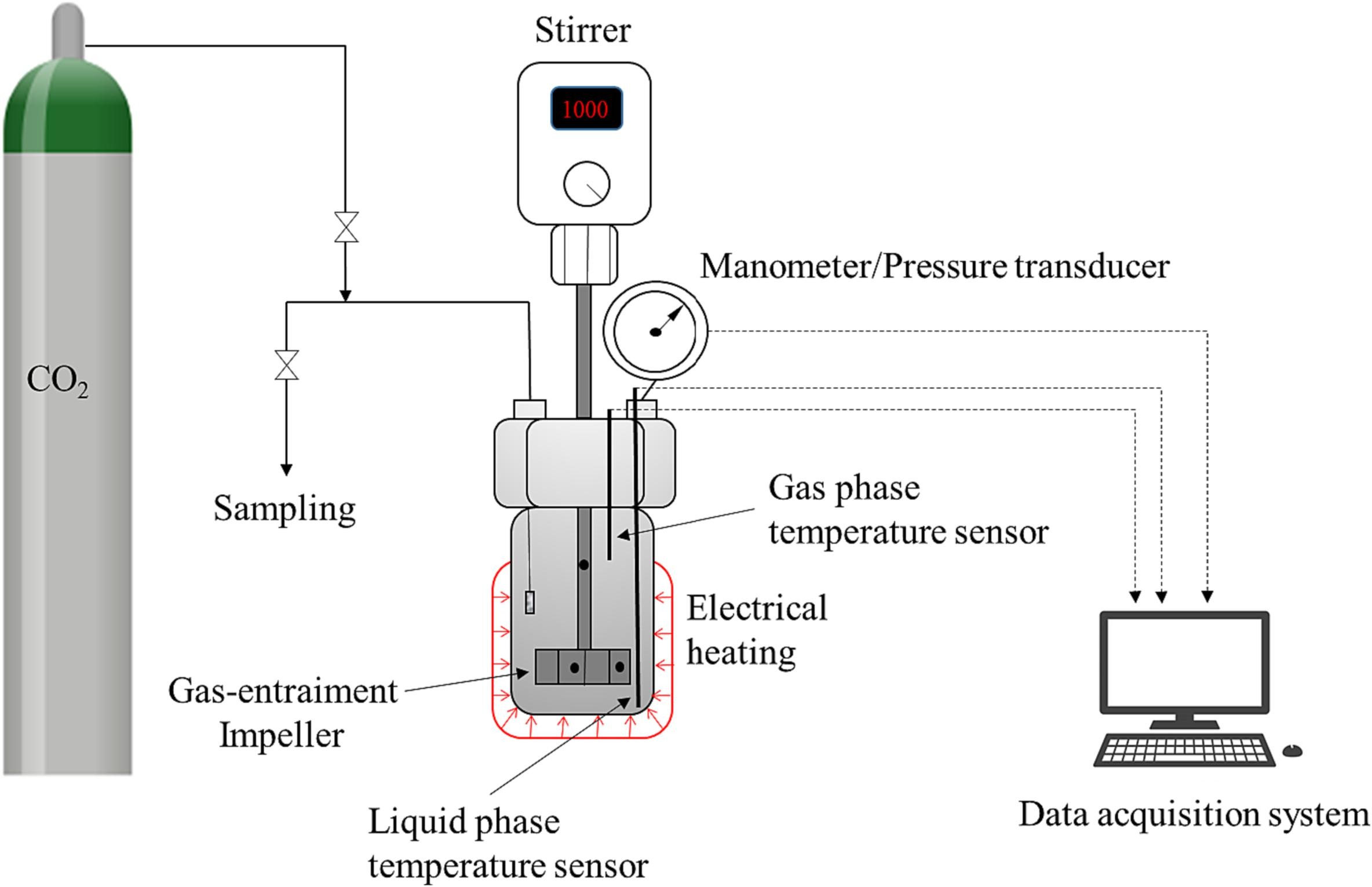
Fatty acid epoxides are obtained by epoxidation of fatty acids and esters with hydrogen peroxide. Carbonation of fatty acid epoxides with CO2 is an important topic in the development of environmentally friendly technologies for the production of chemical intermediates, polymers and fuel components. Very precise kinetic and mass transfer studies on the carbonation of a tall-oil model component, epoxidized methyl oleate were carried out in an autoclave operating at 20–60 bar CO2 and 100–160 °C. SBA-15(0.12)-4PPI was used as the grafted heterogeneous catalyst. The reaction times were 0–23 h and the stirring rates were varied between 200 and 1000 rpm to study the gas–liquid mass transfer of CO2. The main reaction product was the carbonate, while some amounts of ketone were formed as a side product via the Meinwald rearrangement. Five rival reaction mechanisms were considered, based on the concept of interaction between the Lewis and nucleophilic sites on the catalyst surface. A multiphase reactor model including the interaction of intrinsic kinetics and gas–liquid mass transfer effects was developed to determine the parameters from the experimental data. The model reproduced the experimental data very well, giving a perspective for process scale-up.