Kinetic Modeling of Solketal Synthesis from Glycerol and Acetone Catalyzed by an Iron(III) Complex
Taddeo F, Esposito R, Russo V, Di Serio M.
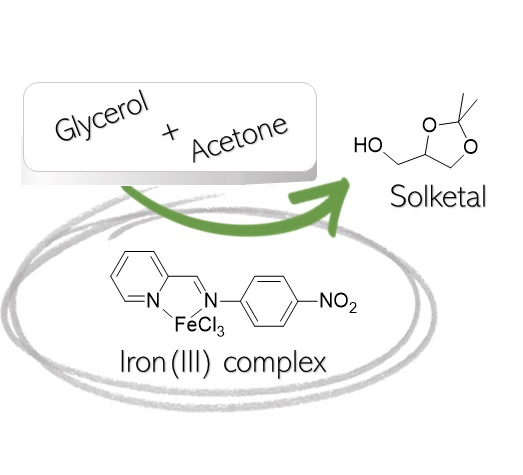
Abstract
In the last few years, the depletion of the fossil sources and their negative effect on the environment has led to find new alternatives; among these, biodiesel is considered one of the most promising for this purpose. Biodiesel can be produced from the transesterification of vegetable oils or animal fats, obtaining glycerol as a by-product. Glycerol can be used in different processes and one of the most interesting is the condensation with acetone to produce solketal. Among its applications, plasticizers, solvents, and pharmaceutical formulations are the most common. In this work, the attention was focused on the reaction between glycerol and acetone to give solketal promoted by an iron(III) complex. The reaction mechanism was hypothesized, and the kinetics was studied in a batch reactor. Finally, the thermodynamic and kinetic parameters were determined with a reliable model investigating the phenomena that occurred in the reaction network.