Isobaric vapour-liquid equilibria for some halogen-containing ethanes in binary mixtures with HF
Santacesaria, E.; Tesser, R.; Di Serio, M.; Basile, G.
Journnl of Fluorfue Ch.erni.strg, 61 (1993) 123-131
https://doi.org/10.1016/S0022-1139(00)80422-4
Abstract
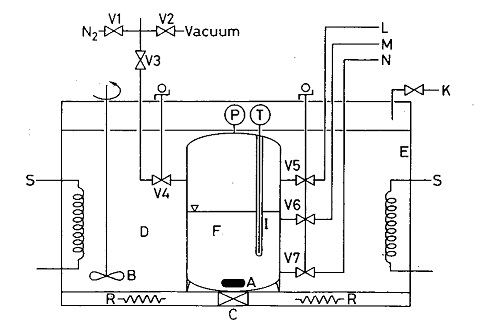
The vapour-liquid equilibria (VLE) of four binary mixtures of HF with CCl2F-CF2Cl, CHCl2-CF3, CH2Cl-CF3 and CH2F-CF3, respectively, have been studied under isobaric conditions. Experimental results have been interpreted by the NRTL (Not Random Two Liquids Theory) method with three parameters. The non-ideality and association of the vapour phase has been described with the virial equation. Despite the strong association of the HF molecules, the NRTL method satisfactorily reproduces the vapour-liquid equilibria of the binary mixtures, especially their lack of miscibility.