Ethylene Oxide Solubility and Ethoxylation Kinetics in the Synthesis of Nonionic Surfactants
Di Serio, M.; Tesser, R.; Felippone, F.; Santacesaria, E.
Ind. Eng. Chem. Res. 1995, 34, 11, 4092–4098
https://doi.org/10.1021/ie00038a052
Abstract
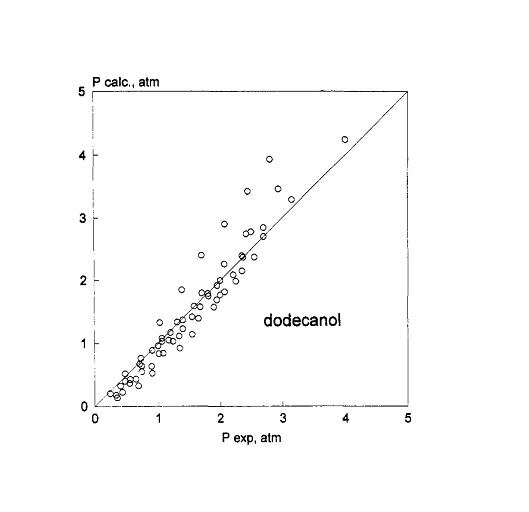
Solubility data from different sources of ethylene oxide in nonylphenol, dodecanol, dodecanoic acid, and their related ethoxylated derivatives have been interpreted by the authors with the UNIFAC, NRTL (nonrandom two-liquid theory), and Wilson methods. The results obtained with these methods have been compared. The two parameters of the NRTL and Wilson equations have been correlated with the mean number of the ethylene oxide adducts of the hydrophilic chain by applying quadratic polynomials. The Wilson equation give the best performances. The role of ethylene oxide solubility in ethoxylation kinetics and mass transfer will be stressed. Kinetic and mass-transfer parameters are clearly correlated with the ethylene oxide concentration. Therefore, the availability of more reliable solubility data or the use of a different method to represent these data requires the recalculation of kinetic parameters. This has been done for the three different substrates considered by applying the solubility data calculated with the Wilson equations. Small differences are observed in the kinetic parameters for dodecanol and dodecanoic acid with respect to previously published data, while remarkable differences are observed for nonylphenol as a consequence of the acquisition of more precise solubility data