Michele Emanuele Fortunato, Rosa Vitiello, Francesco Taddeo, Luigi Faro, Salvatore Mallardo, Rosa Turco, Riccardo Tesser, Vincenzo Russo, Martino Di Serio
European Journal of Lipid Science and Technology, 2025; 0:e202400211
ABSTRACT
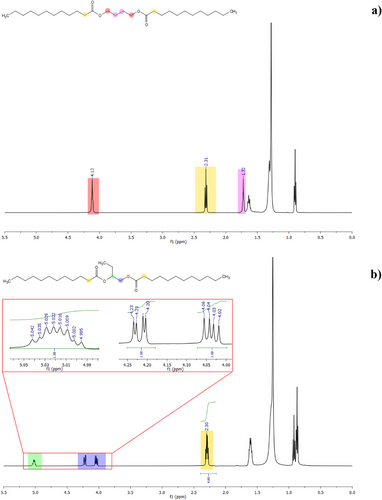
In response to the increasing demand for sustainable materials, this study explores the structure–property relationships of 22 novel diesters synthesized from C2–C4 linear and branched diols and saturated fatty acids (SFAs), targeting the improvement of vegetable oil-based lubricants’ oxidative stability and low-temperature performance. Diesters based on pelargonic (C9), lauric (C12), myristic (C14), and palmitic (C16) acids were characterized for viscosity, crystallization temperature, and flow behavior. Branched diols significantly enhanced low-temperature properties, with 1,2-butanediol and 1,3-butanediol diesters always exhibiting the lowest crystallization temperatures. Viscosity increased with fatty acid chain length, whereas branching caused a slight reduction due to steric hindrance. Most diesters showed shear-thickening behavior, modeled by the Herschel–Bulkley equation. Additionally, all diesters demonstrated excellent oxidative stability, surpassing 300 min in accelerated aging tests. These findings suggest that the synthesized diesters, particularly those with branched diols, offer promise as sustainable, high-performance alternatives for industrial lubricant applications.