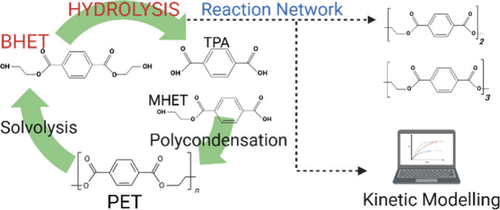
This study investigates the kinetics of bis(2-hydroxyethyl) terephthalate (BHET) hydrolysis catalyzed by Sb2O3, aiming to optimize the chemical recycling process of polyethylene terephthalate (PET) since BHET can be obtained from glycolysis of PET. The hydrolysis of BHET leads to a network of reactions, including hydrolysis, esterification, condensation, transesterification, and dimerization. The reactions were conducted at different temperatures (160–200 °C) and initial water concentrations (2.5–12.5 wt %). Results demonstrated that higher temperatures and water concentrations significantly enhance hydrolysis efficiency, as indicated by increased carboxyl end groups (CEG) values. The study employed mass balance equations and nonlinear regression analysis to estimate kinetic and thermodynamic parameters. The kinetic model was tested via experimental data, showing deviations within ±20%. This comprehensive kinetic analysis provides insights into optimizing hydrolysis conditions to maximize the yield of hydrolyzed BHET, facilitating the subsequent polycondensation process to produce high-quality recycled PET (r-PET).