The kinetic behaviour of b-galactosidase from Kluyveromices marxianus (Saccharomyces) lactis, (Maxilact 5000 Gist-Brocades France S.A. ),immobilized on different oxide supports, such as alumina, silica, and silicated alumina was studied. The activity of the immobilized lactase on different supports was tested by using the ONPG (0-nitrophenyl-b-D-galactopyranoside) hydrolysis test.
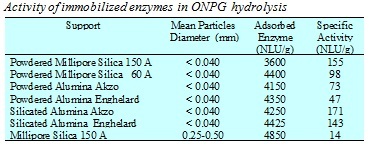
As the enzyme b-galactosidase can be anchored only in pores with adiameter greater than 150 Å the pore distribution of the supports has a strong influence on the immobilized enzyme activity. Also the chemical nature of the support seems to have a significant influence on the activity of the anchored enzyme. Silica is, for example, a better support than alumina.
Moreover, the enzyme anchored on silicated alumina, that is, alumina coated with SiO2 by grafting silicon alkoxide on the alumina surface and calcinating, shows greater activity compared to the one obtained with the original alumina support. However, the most important characteristic of the support is the mean diameter of the particles. Immobilized enzyme activity strongly decreases as the particle diameter of the support increases. But, for practical purposes, in both fixed and fluidized bed reactors, we need particles of acceptable size and so it is necessary to accept a compromise between activity and particle size.
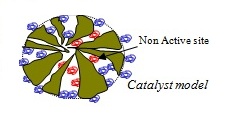
We have experimentally observed an effectiveness factor of about 0.1 for particles of 0.25-0.5 mm diameter, but we believe that the calculation of the effectiveness factor cannot be made using the traditional approach and needs the development of a more sophisticated model because the internal structure of these pores is strongly modified as a consequence of the enzyme anchoring. Bulky enzyme molecules fill the large pores preventing the diffusion of reagent and product molecules inside the catalytic particles. Moreover, enzyme molecules grafted on the solid surface are not all active and it is highly likely that active sites may not be uniformly distributed inside the pores.
Kinetic runs of lactose hydrolysis were performed by using a continuous packed bed tubular reactor containing the enzyme immobilized on silica spheres of 0.25-0.50 mm. The runs were performed by changing: feed rates, reagent and product concentrations and temperatures. The kinetic data show a depressing effect of the products on hydrolysis reaction rates that can be described by the reaction mechanism. The proposed model describes all the performed runs quite well (mean error 12%, correlation index 0.997).
